ABSTRACT
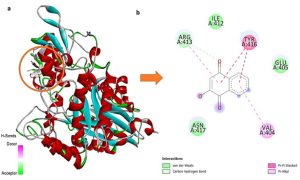
Background: Our previous research highlighted remarkable hypoglycemic and hypolipidemic potentials of lawsone methyl ether (LME or 2-methoxy-1,4-naphthoquinone) and lawsone (2-hydroxy-1,4-naphthoquinone) in diabetic rats via β-cell regeneration. This insighted us to explore their additional antidiabetic mechanisms against α-glucosidase using in silico and in vitro approaches. Materials and Methods: In silico molecular docking was performed via Autodock Vina, SwissADME, and Datawarrior software. However, an in vitro inhibitory assay was conducted against α-glucosidase. Results: In silico studies revealed promising binding conformations and interactions of LME and lawsone with the functional residues of the α-glucosidase protein, involving hydrogen bonding, Van der Waals, and pi-pi interactions, showing comparable binding energies of -5.4 and -5.6 kcal/mol, respectively. Additionally, LME and lawsone displayed favorable pharmacokinetic profiles, revealing no evident toxicity. In vitro α-glucosidase inhibitory assay indicated that LME (IC50 of 37.4 μg/mL) and lawsone (IC50 of 42.2 μg/mL) exhibited comparable inhibitory activities, while both of them possessed markedly higher activities than acarbose (IC50 of 440.6 μg/mL). Furthermore, study on synergistic effects among these naphthoquinones and acarbose illustrated that at ½IC50 of LME (18.7 μg/mL) and acarbose (220.3 μg/mL) exhibited a satisfactory synergistic effect against α-glucosidase, with a percentage inhibition of 88.7% and a fractional percentage inhibition index (FPI) of 2.0, while at ½IC50 of lawsone (21.1 μg/mL) and acarbose (220.3 μg/mL) produced an additive effect, with a percentage inhibition of 76.4% and a FPI of 1.7. Conclusion: Promising α-glucosidase inhibitory potentials of LME and lawsone underscore their additional mechanism alongside β-cell regeneration further supporting their outstanding antidiabetic capabilities.
