ABSTRACT
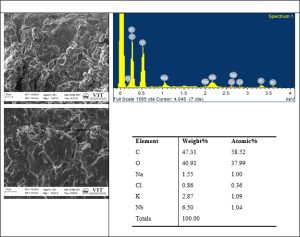
Background: Santha Chandrodaya Mathirai (SSM) [Cāntacantirōtaya māttirai] is a classical tablet formulation in Siddha medicine used in various types of fevers. Because of limited research validation in terms of the product compactness, quality and safety, the present study focuses on the formulation, characterization and standardization of the tablet dosage. The objective of the study is to prepare SSM as per Standard operating Procedures (SoPs) mentioned in classical text and to characterize it chemically using modern analytical techniques. Materials and Methods: The tablet dosage was prepared from the In-house R&D GMP Pharmacy facility of Siddha central research Institute and validated through analytical measures like pre-compression, post-compression parameters, physiochemical analysis, analytical studies like High Performance Thin Layer Chromatography (HPTLC), Special Edition Microscopy (SEM) with Energy Dispersive X-Ray (EDAX), Fourier Transform Infrared Spectroscopy (FT-IR) and UV Absorption Spectroscopy (UV-AS). Results: As per the reference standards, the mean flow property of the Tablet granules (31˚) was fair enough, the mean compressibility index (17.3%) and Hausner’s ratio (1.452) indicates its good flow character. The tablet passed the USP standards of weight variation in %. The friability test reported the maximum weight loss to be 0.06 %, a good acceptable value. The highest disintegration time was observed at 60 min. The samples were devoid of Heavy metals, microbial and aflatoxin contamination. At short UV of 254 nm, long UV of 366 nm, and post derivatized plate in white light there were observation of 6 spots, 8 spots and 12 spots respectively in TLC photo documentation. EDAX reported the presence of Carbon, Oxygen, Sodium, Chlorine, Potassium, and Niobium molecules in the sample with no traces of heavy metals. FTIR spectra showed three high peak areas at the range of band 2917.08, 2849.10, and at 1717.08 that corresponds to carboxylic acid, alkane bond, and α, β-unsaturated ester. Conclusion: The In-house samples of SSM reported standard values in terms of quality and safety. Further clinical trials are warranted to validate its efficacy.
