ABSTRACT
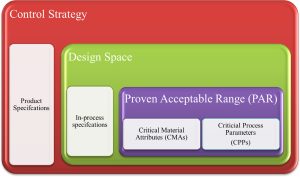
The Quality by Design (QbD) concept has been appreciated and expected by the regulatory agencies, especially the “United States Food and Drug Administration” (USFDA), the “European Medicines Agency” (EMA), and other agencies that have adopted the “International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use” (ICH) around the globe. This paper describes the application of QbD principles in the pharmaceutical development of Oral Solid Dosage forms (OSDs). It encourages the implementation of risk-based approaches when designing pharmaceutical products. It provides a thorough understanding of formulation variables, Critical Material Attributes (CMAs), process variables, and Critical Process Parameters (CPPs) based on industrial experience that can be considered for risk assessment during the development of OSDs. It provides handy guidance to academics, research scholars, and industry scientists for implementing QbD in developing the OSDs.
